
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
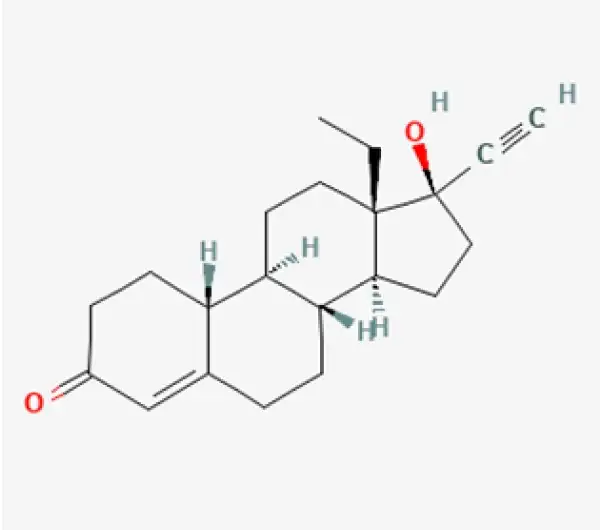
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Levonorgestrel
C₂₁H₂₈O₂
~312.45 g/mol
797-63-7
(13S,14S,17R)-13-ethyl-17-hydroxy-11-methylene-18,19-dinor-17α-pregn-4-en-20-yn-3-one
Synthetic progestin
Contraceptive
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in ethanol, chloroform, and methanol |
| Melting Point | 232–234°C |
| pH | - |
Levonorgestrel is a synthetic progestin widely used for hormonal contraception and emergency contraceptive therapy. It acts by preventing ovulation, thickening cervical mucus, and altering the endometrial lining, thereby reducing the likelihood of fertilization and implantation. It is orally active, highly potent at low doses, and forms the active component of many contraceptive pills, intrauterine systems, and emergency contraceptive products.
Levonorgestrel works by suppressing ovulation through inhibition of the luteinizing hormone (LH) surge. It also increases cervical mucus viscosity, which prevents sperm from reaching the egg, and alters endometrial receptivity, reducing the likelihood of implantation. Additionally, it exhibits high receptor selectivity, minimizing androgenic and estrogenic side effects while effectively providing contraceptive action.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, it is widely used for tablets, capsules, and intrauterine systems due to its high potency and oral bioavailability.
Yes, all regulatory documentation is supplied with every batch for export and validation purposes.
Yes, the API is produced in fully cGMP-compliant facilities with validated quality control methods.
Yes, it retains its progestogenic activity, effectively suppressing ovulation and modulating endometrial receptivity, while stability studies confirm its suitability for long-term storage and formulation.
Looking to source Levonorgestrel or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.