
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP/BP
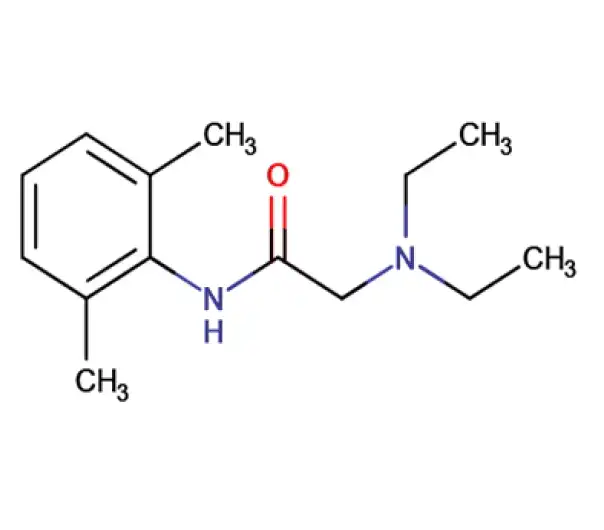
C14H22N2O
2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide
137-58-6
234.33 g/mol
amide-type
Local Anesthetic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water (as hydrochloride salt), slightly soluble in alcohol, practically insoluble in ether (base form) |
| Melting Point | 68–69 °C |
| pH | 5.0-7.0 |
Lidocaine is a widely used local anesthetic and antiarrhythmic agent. It works by blocking sodium channels in nerve cells, preventing the transmission of pain signals, which results in temporary numbness in the targeted area. Lidocaine is commonly applied topically or injected for minor surgical procedures, dental work, and other medical interventions requiring localized anesthesia. Additionally, it is used intravenously to treat certain types of irregular heartbeats (arrhythmias). Known for its rapid onset and moderate duration of action, lidocaine is considered a safe and effective medication when used appropriately.

Lidocaine works by blocking voltage-gated sodium channels on nerve cell membranes. This inhibition prevents the initiation and conduction of nerve impulses, stopping pain signals from traveling to the brain and causing localized numbness. When used as an antiarrhythmic, lidocaine stabilizes the heart’s electrical activity by suppressing abnormal impulses, helping to restore normal heart rhythm.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Lidocaine is used to numb tissues for minor surgeries, dental procedures, and to treat certain heart rhythm problems.
It typically takes effect within minutes after application or injection.
Lidocaine is generally considered safe when used appropriately during pregnancy but always consult a healthcare provider first.
Yes, Salius Pharma Pvt. Ltd. exports Lidocaine API to several countries across Africa and the Middle East. Their export activities encompass regions such as Saudi Arabia, Ethiopia, Uzbekistan, Afghanistan, and Somalia, among others. The company is recognized as a Government of India-accredited Star Export House and adheres to stringent WHO-GMP, USFDA, UK MHRA, and TGA Australia certifications, ensuring compliance with international quality standards.
Yes, Salius Pharma provides full technical support and all necessary regulatory documentation for Lidocaine API, including DMFs, CEPs, and CTD dossiers. They ensure compliance with global standards to facilitate smooth product registration and export Lidocaine API to African and Middle Eastern countries.
Looking to source Lidocaine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.