
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
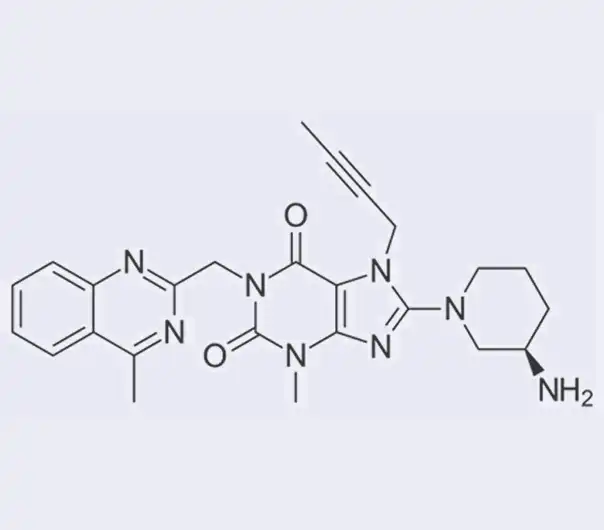
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Linagliptin
C₂₅H₂₈N₈O₂
~472.55 g/mol
668270-12-0
8-[(3R)-3-aminopiperidin-1-yl]-7-(but-2-yn-1-yl)-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-3,7-dihydro-1H-purine-2,6-dione
Xanthine derivative
Antidiabetic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in DMSO, methanol, and ethanol |
| Melting Point | 295–298°C |
| pH | - |
Linagliptin is an oral antidiabetic agent that selectively inhibits dipeptidyl peptidase-4 (DPP-4), an enzyme responsible for the breakdown of incretin hormones like GLP-1. By prolonging incretin activity, it enhances glucose-dependent insulin secretion and suppresses glucagon release, helping to maintain blood glucose levels in patients with type 2 diabetes mellitus. Linagliptin offers the advantage of once-daily dosing without dose adjustment in mild-to-moderate renal impairment.
Linagliptin selectively inhibits DPP-4, preventing the degradation of incretin hormones such as GLP-1 and GIP. This action enhances glucose-dependent insulin secretion from pancreatic β-cells and suppresses glucagon secretion, thereby reducing hepatic glucose production. When used as monotherapy, it does not cause significant hypoglycemia, making it a safe and effective option for managing type 2 diabetes.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, widely used in tablets and fixed-dose combinations for type 2 diabetes.
Yes, all documentation is supplied for export and regulated markets.
Yes, fully cGMP-compliant with validated QC methods.
Yes, custom particle sizes and grades are available to optimize dissolution and formulation.
Yes, long-term and accelerated stability studies confirm API stability for tropical and temperate climates.
Looking to source Linagliptin or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.