
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP/BP/USP/EP
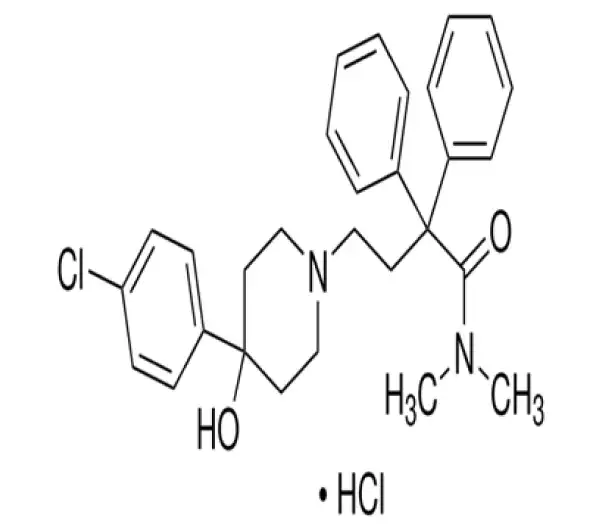
C29H33ClN2O2 · HCl
4-(4-Chlorophenyl)-4-hydroxy-N,N-dimethyl-α,α-diphenyl-1-piperidinebutanamide hydrochloride
34552-83-5
513.50 g/mol
Piperidine derivative
Anti-Diarrheal
| Appearance | White to slightly yellowish crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; freely soluble in methanol, ethanol, and chloroform |
| Melting Point | 222–225°C |
| pH | 4.0–5.0 |
Loperamide HCl is a synthetic antidiarrheal agent classified as a piperidine derivative. It works by acting on μ-opioid receptors in the intestinal wall to reduce peristalsis and prolong transit time, allowing more water and electrolytes to be absorbed. Unlike other opioids, it does not significantly cross the blood-brain barrier, so it lacks central nervous system effects at therapeutic doses. Loperamide is commonly used to treat acute and chronic diarrhea, including traveler’s diarrhea and diarrhea associated with irritable bowel syndrome (IBS). It is widely known under the brand name Imodium and is available in various oral dosage forms. Its efficacy and low risk of central opioid side effects make it a first-line agent for symptomatic diarrhea control.
Loperamide Hydrochloride works by activating opioid receptors (specifically μ-opioid receptors) in the intestinal wall, which slows down intestinal motility (peristalsis). This slower movement allows more time for water and electrolytes to be absorbed back into the body, resulting in firmer stools and reduced frequency of diarrhea. Importantly, loperamide acts mostly locally in the gut and does not cross the blood-brain barrier significantly, so it doesn’t cause central opioid effects like pain relief or addiction at normal doses.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It’s used to treat acute and chronic diarrhea by slowing intestinal movement.
Effects are usually seen within 1–2 hours after taking the dose.
At recommended doses, it does not cause addiction because it doesn’t cross the blood-brain barrier significantly.
Yes, Salius Pharma Pvt. Ltd. exports Loperamide Hydrochloride (HCl) API to various countries, including those in Africa and the Middle East. Their global export activities encompass a wide range of pharmaceutical products, including APIs, to numerous international markets. While specific details about Loperamide HCl shipments are not publicly disclosed, their extensive export operations suggest that Loperamide HCl is part of their product offerings in these regions.
Yes, Salius Pharma provides technical support and regulatory documentation for Loperamide HCl API, including Drug Master Files, Certificates of Suitability, and clinical data to help with product registration. They work closely with clients to tailor documentation and assist with regulatory approvals, especially for markets in Africa and the Middle East, ensuring compliance with international standards.
Looking to source Loperamide HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.