
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
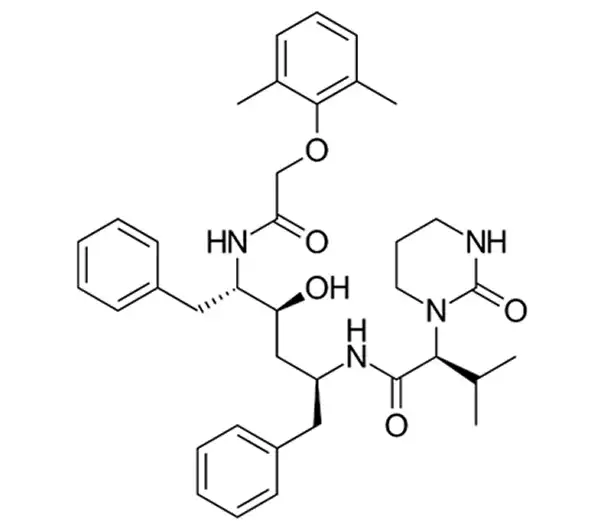
Lopinavir
C₃₇H₄₈N₄O₅
~628.8 g/mol
192725-17-0
(2S)-N-[(2S,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetyl]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxopyrrolidin-1-yl)butanamide
HIV-1 protease inhibitor
Antiretroviral
| Appearance | White to almost white crystalline powder / solid |
|---|---|
| Solubility | Practically insoluble in water; soluble in methanol, ethanol, DMSO |
| Melting Point | ~124–126°C (decomposition) |
| pH | - |
Lopinavir is a potent HIV-1 protease inhibitor used in combination therapy with Ritonavir to treat HIV infection. It inhibits the HIV-1 protease enzyme, preventing cleavage of viral polyproteins and formation of mature infectious virions. Lopinavir provides high antiviral activity, favorable pharmacokinetics when boosted with Ritonavir, and is widely used in both treatment-naive and experienced patients.
1. Inhibits HIV-1 protease, preventing cleavage of Gag-Pol polyproteins.
2. Results in production of immature, non-infectious viral particles.
3. Boosted with Ritonavir to increase plasma concentrations via CYP3A4 inhibition.
4. Reduces viral load and improves CD4+ T-cell counts in infected patients.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, widely used with Ritonavir or other antiretrovirals in oral tablets and capsules.
Yes, all documentation is supplied for export and regulatory compliance in LATAM, MENA, Africa, and Asia.
Yes, fully cGMP-compliant with validated quality control methods.
Yes, custom grades are available to optimize formulation and dissolution characteristics.
Yes, long-term and accelerated stability studies confirm API integrity under recommended storage conditions.
Looking to source Lopinavir or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.