
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP
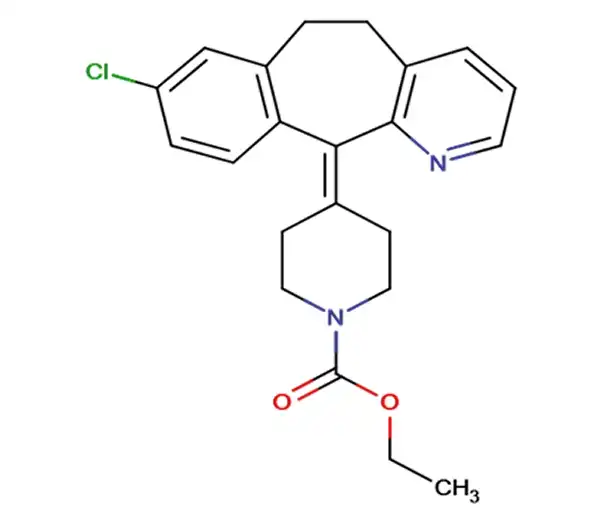
C22H23ClN2O2
ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate
79794-75-5
382.88 g/mol
Tricyclic piperidine derivative
Anti-Histamine
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in ethanol, methanol, and chloroform |
| Melting Point | 134–136°C |
| pH | 6–7 |
Loratadine is a second-generation antihistamine widely used to relieve symptoms of allergic conditions such as hay fever (allergic rhinitis) and chronic urticaria (hives). It works by selectively blocking peripheral H1 histamine receptors, which helps reduce symptoms like sneezing, itching, watery eyes, and runny nose without causing significant drowsiness. Loratadine is preferred over first-generation antihistamines due to its minimal sedative effects and longer duration of action. It is commonly available in oral tablets, syrups, and rapidly disintegrating formulations, making it a popular choice for allergy management worldwide.

Loratadine works by selectively blocking H1 histamine receptors located on cells in the body’s tissues. By preventing histamine—a chemical released during allergic reactions—from binding to these receptors, loratadine reduces allergy symptoms such as sneezing, itching, runny nose, and watery eyes. Because it primarily acts on peripheral H1 receptors and does not easily cross the blood-brain barrier, loratadine causes minimal sedation compared to first-generation antihistamines.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, loratadine is a second-generation antihistamine designed to cause minimal drowsiness.
Loperamide typically starts working within 1 to 2 hours after oral administration, helping to reduce diarrhea and improve stool consistency.
Generally yes, but inform your healthcare provider about all the medicines you are taking to avoid interactions.
Yes, Salius Pharma Pvt. Ltd. exports Loratadine API to various regions, including Africa and the Middle East. As a Government of India-accredited Star Export House, Salius Pharma has a strong export presence in over 50 countries. Their product portfolio includes a wide range of Active Pharmaceutical Ingredients (APIs), and they are committed to delivering high-quality, cost-effective healthcare solutions worldwide.
Yes, Salius Pharma Pvt. Ltd. offers comprehensive technical support and regulatory documentation for its Loratadine API, facilitating product registration and importation in various international markets, including Africa and the Middle East.
Looking to source Loratadine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.