
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP / BP / USP
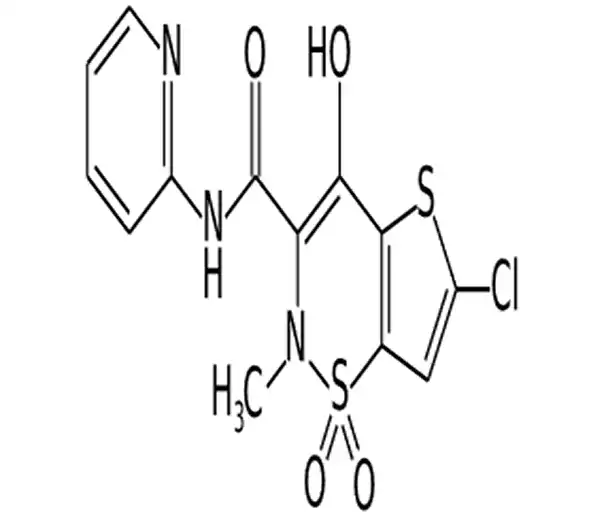
C13H10ClN3O4S2
6-chloro-4-hydroxy-2-methyl-N-(2-pyridyl)-2H-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide
70374-39-9
371.82 g/mol
Non-Steroidal Anti-Inflammatory Drug (NSAID)
Analgesic / Anti-inflammatory agent
| Appearance | Yellow to yellow-orange crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in methanol, ethanol, and alkaline solutions |
| Melting Point | 220–230°C |
| pH | Neutral to slightly acidic depending on solvent system |
Lornoxicam is a potent oxicam-class non-steroidal anti-inflammatory drug (NSAID) with strong analgesic and anti-inflammatory activity. It is widely used in the treatment of acute and chronic pain conditions such as rheumatoid arthritis, osteoarthritis, and postoperative pain. Its balanced inhibition of COX-1 and COX-2 enzymes provides effective relief with an improved gastrointestinal tolerance profile compared to traditional NSAIDs.
Lornoxicam acts by inhibiting the cyclooxygenase enzymes (COX-1 and COX-2), thereby blocking prostaglandin synthesis — the mediators responsible for pain, swelling, and inflammation. This mechanism provides effective analgesic and anti-inflammatory effects across various conditions while minimizing the risk of gastric irritation.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Lornoxicam is used for treating acute and chronic pain, including musculoskeletal pain, rheumatoid arthritis, osteoarthritis, and postoperative conditions.
Yes. Salius Pharma supplies high-purity Lornoxicam API to formulation manufacturers worldwide with full documentation support.
Yes. We export to Asia, the Middle East, Europe, Africa, CIS countries, and South America with regulatory compliance and logistics support.
Yes. DMF (Open Part), COA, MSDS, and CTD dossiers are available for submission in regulated and semi-regulated markets.
Yes. Custom purity grades or particle size specifications can be developed based on formulation requirements or regulatory needs.
Standard export packing in HDPE or fiber drums with double liners, nitrogen-flushed if required for long-distance shipments.
Looking to source Lornoxicam or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.