
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
PN*
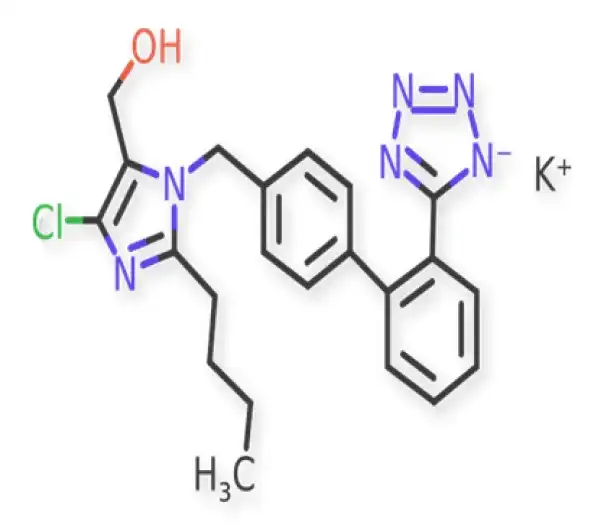
C22H22ClN6O.K
1H-Imidazole-5-methanol, 2-butyl-4-chloro-1-((2'-(1H-tetrazol-5-yl)(1,1'-bi phenyl)-4-yl)methyl)-, monopotassium salt
124750-99-8
461 g/mol
Biphenyl-tetrazole derivative
Anti-Hypertensive
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in methanol and ethanol |
| Melting Point | 250–255°C |
| pH | 4.5–6.5 |
Losartan Potassium is an oral antihypertensive drug belonging to the angiotensin II receptor blocker (ARB) class. It works by selectively blocking the AT₁ receptors, which prevents the action of angiotensin II, a hormone that causes blood vessels to constrict. This leads to vasodilation, reduced blood pressure, and decreased workload on the heart. Losartan is commonly prescribed for managing hypertension, protecting kidney function in diabetic patients, treating heart failure, and reducing the risk of stroke in patients with left ventricular hypertrophy. It is well-tolerated and often preferred for patients who cannot use ACE inhibitors due to side effects like cough.

Losartan Potassium works by selectively blocking the angiotensin II type 1 (AT₁) receptors found on blood vessels and various tissues. By preventing angiotensin II—a powerful vasoconstrictor—from binding to these receptors, losartan causes blood vessels to relax and widen (vasodilation). This reduces blood pressure, decreases the workload on the heart, and helps protect organs such as the kidneys from damage caused by high blood pressure.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, Losartan can be taken with or without food.
No, Losartan is contraindicated during pregnancy due to risks to the fetus.
It usually starts lowering blood pressure within 1 hour, with full effects seen after 3 to 6 weeks.
Yes, Salius Pharma Pvt. Ltd. exports Losartan Potassium API to various regions, including Africa and the Middle East. As a Government of India-accredited Star Export House, Salius Pharma has a strong export presence in over 50 countries. Their product portfolio includes a wide range of Active Pharmaceutical Ingredients (APIs), and they are committed to delivering high-quality, cost-effective healthcare solutions worldwide.
Yes, Salius Pharma Pvt. Ltd. offers comprehensive technical support and regulatory documentation for its Losartan Potassium API, facilitating product registration and importation in various international markets, including Africa and the Middle East.
Looking to source Losartan Potassium or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.