
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP/BP
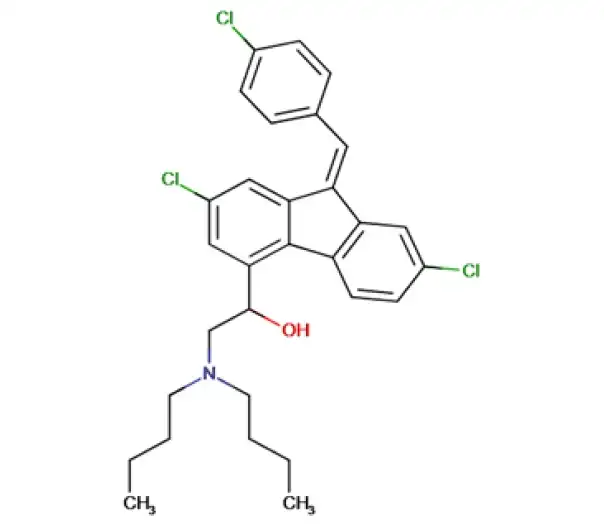
C30H32Cl3NO
2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-[(4-chlorophenyl)methylidene]-9H-fluoren-4-yl]ethan-1-ol
82186-77-4
528.94 g/mol
Fluorene derivative
Anti-Malarial
| Appearance | Yellow to orange crystalline powder |
|---|---|
| Solubility | Soluble in methanol, ethanol, dichloromethane, and chloroform; very slightly soluble in acetonitrile |
| Melting Point | 130–132°C |
| pH | - |
Lumefantrine is a synthetic arylaminoalcohol antimalarial drug used in combination therapy to treat uncomplicated malaria caused by Plasmodium falciparum. It is most commonly used in fixed-dose combination with artemether (as artemether-lumefantrine, e.g., Coartem).

Lumefantrine works by inhibiting the formation of hemozoin, a detoxified form of heme produced by the parasite during hemoglobin digestion. By blocking this process, toxic free heme accumulates, damaging the parasite's membranes and leading to its death. Lumefantrine acts more slowly than artemether but has a longer half-life, helping to eliminate residual parasites and prevent recurrence. Its absorption is significantly enhanced when taken with fatty food.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used with artemether to treat uncomplicated Plasmodium falciparum malaria.
It should be taken orally with a fatty meal to improve absorption.
No, it is always used in combination with artemether to prevent resistance.
Yes, Salius Pharma Pvt. Ltd., based in Navi Mumbai, exports Lumefantrine API to various countries, including those in Africa and the Middle East. Their export activities encompass a wide range of pharmaceutical products, including APIs, to numerous global markets. While specific details about Lumefantrine shipments are not publicly disclosed, their extensive export operations suggest that Lumefantrine is part of their product offerings in these regions.
Yes, Salius Pharma offers comprehensive technical support and regulatory documentation for Lumefantrine API. They provide essential documents such as DMF, CEP, CTD dossiers, and bioequivalence data to facilitate product registration and compliance with international standards like WHO GMP, USFDA, and EU regulations.
Looking to source Lumefantrine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.