
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/EP
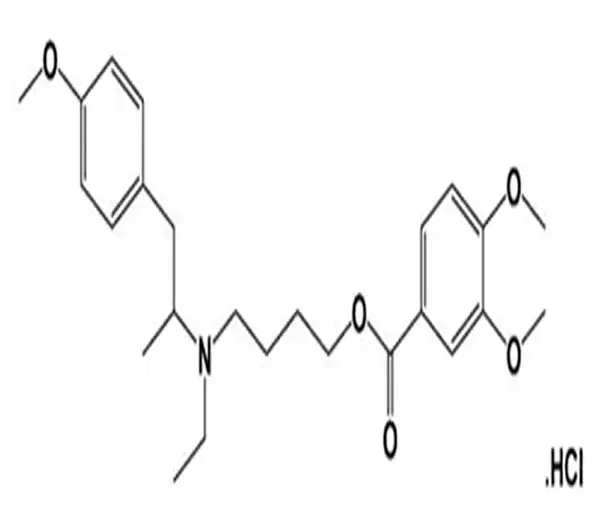
C25H36ClNO5
4-(ethyl(p-methoxy-alpha-methylphenethyl)amino)butyl ester, hydrochloride
2753-45-9
466.07 g/mol
Mebeverine
Anti-Spasmodic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in alcohol and chloroform |
| Melting Point | 140–145°C |
| pH | 5–6 |
Mebeverine HCl is an antispasmodic medication used to relieve muscle spasms in the gastrointestinal tract. It works by directly relaxing the smooth muscles of the gut without affecting normal bowel movements. This makes it effective in treating symptoms of irritable bowel syndrome (IBS), including abdominal pain, cramping, and discomfort. Mebeverine helps improve gut motility and reduces spasms, offering symptomatic relief without causing sedation or affecting the central nervous system. It is commonly prescribed worldwide under various brand names and is generally well-tolerated with a low incidence of side effects.
Mebeverine Hydrochloride works by directly relaxing the smooth muscles of the gastrointestinal tract. It acts as a musculotropic antispasmodic, reducing muscle spasms and cramps without affecting normal gut motility or causing sedation. This helps relieve abdominal pain and discomfort associated with conditions like irritable bowel syndrome (IBS).

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used to relieve abdominal pain and muscle spasms in conditions like irritable bowel syndrome (IBS).
Usually taken as a 135 mg tablet three times daily, preferably 20 minutes before meals, or as prescribed by your doctor.
Relief can be noticed within a few days, but full benefit may take 1–2 weeks.
Yes, Salius Pharma Pvt. Ltd. exports Mebeverine Hydrochloride (HCl) API to various international markets, including countries in Africa and the Middle East. As a Government of India-recognized Star Export House, Salius Pharma has a strong global presence and supplies a wide range of pharmaceutical raw materials to these regions.
Yes, Salius Pharma Pvt. Ltd. provides comprehensive technical support and regulatory documentation for Mebeverine Hydrochloride (HCl) API. Their Regulatory Affairs team offers essential documents such as Drug Master Files (DMF), Certificate of Suitability (CEP), clinical trial data, bioequivalence/bioavailability studies, impurity profiles, and technical dossiers prepared according to international guidelines like ACTD and CTD. This ensures smooth and efficient regulatory submissions for their clients worldwide. For more information or to request documentation, customers can contact Salius Pharma directly via email, phone, or their official website.
Looking to source Mebeverine HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.