
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP / EP (as required)
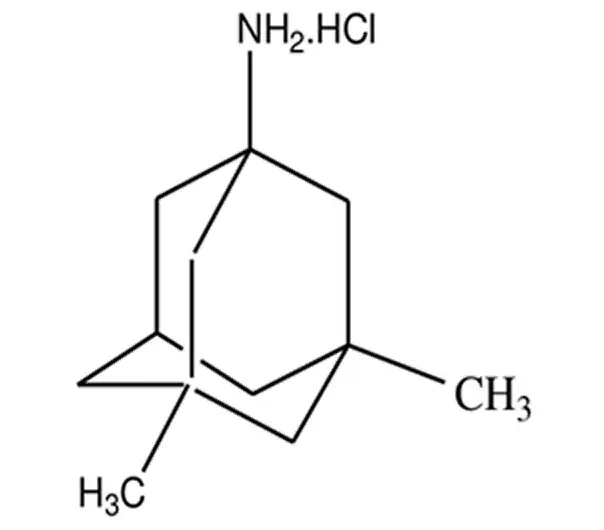
C12H21N·HCl
1-amino-3,5-dimethyladamantane hydrochloride
41100-52-1
231.78 g/mol
Anti-dementia / Cognitive enhancer
| appearance | White to off-white crystalline powder |
|---|---|
| solubility | Freely soluble in water; slightly soluble in alcohol; practically insoluble in organic solvents |
| melting_point | 292–298°C (decomposes) |
| ph | 5.0 – 6.5 |
NMDA receptor antagonist used for moderate-to-severe Alzheimer’s disease; modulates glutamatergic activity to improve cognition and slow symptom progression.
Blocks excessive NMDA receptor stimulation by glutamate, reducing excitotoxicity, protecting neurons, and supporting cognitive function.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
High-purity Memantine Hydrochloride for pharma manufacturing.
Usually 7–21 working days depending on batch and docs.
Yes—Asia, Middle East, Europe, Africa, CIS, South America.
Treatment of moderate-to-severe Alzheimer’s disease.
Often combined with donepezil.
Contact via email/phone/WhatsApp with quantity, destination, docs.
Looking to source Memantine Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.