
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Sterile Injectable API
USP / EP / BP / Injectable Grade
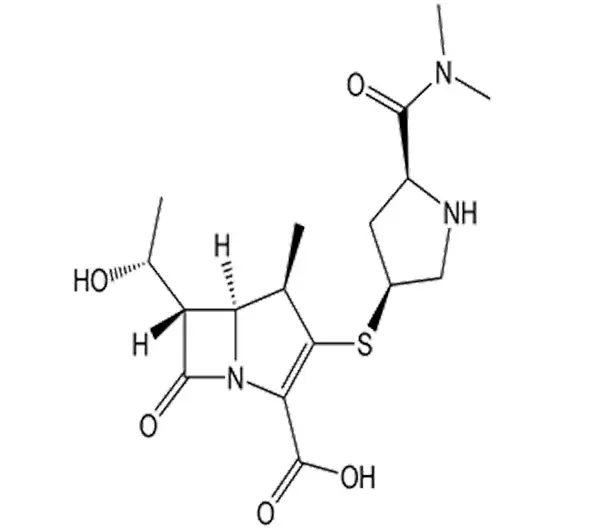
C17H25N3O5S·H2O
(4R,5S,6S)-3-[(3S,5S)-5-(Dimethylcarbamoyl)pyrrolidin-3-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
96036-03-2
383.46 g/mol
Carbapenem Antibiotic
| appearance | White to light yellow lyophilized powder |
|---|---|
| solubility | Freely soluble in water. Reconstituted solution should be used immediately. |
| melting_point | 128–131°C |
| ph | 7.3 – 8.3 |
Sterile, broad-spectrum carbapenem for severe hospital infections; lyophilized for IV use; active against Gram-positive, Gram-negative, and MDR organisms including Pseudomonas and ESBL producers.
Binds PBPs to inhibit bacterial cell wall synthesis, causing lysis; stable to most beta-lactamases, maintaining activity against resistant pathogens.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Severe infections including meningitis, pneumonia, sepsis, intra-abdominal and skin infections.
Yes—covers Gram-positive, Gram-negative, and drug-resistant bacteria.
Typically 24–36 months under controlled storage.
Store below 25°C; protect from moisture and light.
IV injection/infusion after reconstitution with sterile diluent.
Contact with quantity, packaging, and documentation requirements.
Looking to source Meropenem for Injection or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.