
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / EP / BP (as required)
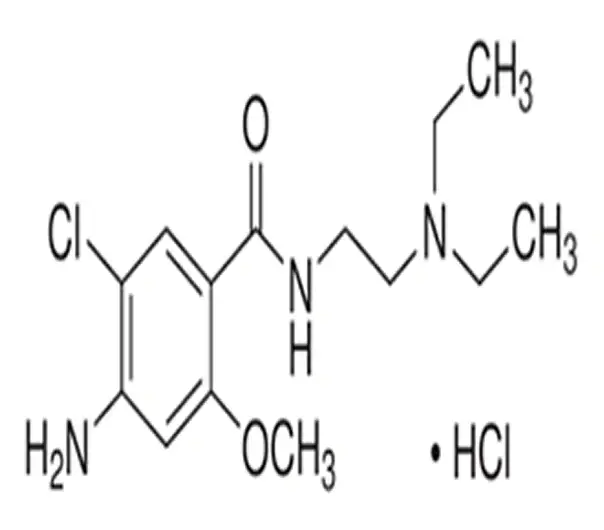
C14H22ClN3O2 · HCl
4-amino-5-chloro-N-(2-(diethylamino)ethyl)-2-methoxybenzamide hydrochloride
54143-57-6
354.30 g/mol
Antiemetic / Prokinetic
| appearance | White or almost white crystalline powder |
|---|---|
| solubility | Freely soluble in water; Soluble in alcohol; Practically insoluble in methylene chloride |
| melting_point | 179–183°C |
| ph | 4.5 – 6.0 (1% aqueous solution) |
Metoclopramide Hydrochloride is an antiemetic and gastrointestinal prokinetic API used for nausea, vomiting, delayed gastric emptying, diabetic gastroparesis, and GERD-related complications. It enhances gastric motility and accelerates gastric emptying without affecting gastric secretions.
Primary mechanisms include D2 receptor antagonism in the CTZ (antiemetic), 5-HT4 receptor agonism (increased acetylcholine release leading to enhanced motility and peristalsis), and mild 5-HT3 antagonism for additional antiemetic effect.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
BP, USP, and EP grades. Each batch undergoes stringent QC to ensure compliance with regulatory standards in regulated and semi-regulated markets.
Yes. COA, MSDS, TDS, stability data, DMF support (where applicable), and commercial documents for customs clearance.
Typically 7–14 business days depending on stock and documentation. Larger orders or PSI requirements may add time.
GI obstruction or perforation, Parkinson’s disease, pheochromocytoma, and history of tardive dyskinesia. Labels in regulated markets must include the required warnings.
Yes. Support for EU, Asia, Africa, Latin America, and Middle East submissions including DMF assistance, impurity profiling, and dossier preparation.
Looking to source Metoclopramide Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.