
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
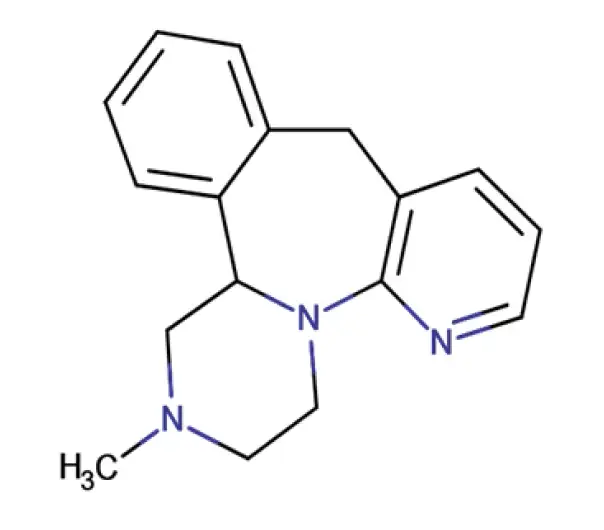
API
PN*
C17H19N3
5-methyl-2,5,19-triazatetracyclo[13.4.0.0^{2,7}.0^{8,13}]nonadeca-1(15),8,10,12,16,18-hexaene
85650-52-8
265.35 g/mol
Tetracyclic Antidepressant
Anti-Depressant
| appearance | White to creamy-white crystalline powder |
|---|---|
| solubility | Slightly soluble in water; Soluble in methanol, ethanol, and chloroform |
| melting_point | 114–116°C |
| ph | 4.5 – 6.5 |
Mirtazapine is a tetracyclic antidepressant primarily used to treat major depressive disorder (MDD). It enhances central noradrenergic and serotonergic activity by antagonizing presynaptic \u03b12-adrenergic receptors and selective serotonin receptors. Unlike SSRIs, Mirtazapine boosts neurotransmitter release without inhibiting serotonin reuptake, making it effective for patients with depression accompanied by sleep disturbances or appetite loss. Its sedative properties also benefit those with anxiety or insomnia.

Mirtazapine blocks presynaptic \u03b12-adrenergic receptors, enhancing norepinephrine and serotonin release. It also antagonizes 5-HT2 and 5-HT3 receptors, increasing serotonin activity at 5-HT1 receptors, which improves mood and reduces anxiety. Its antihistaminic (H1) action contributes to its sedative effect.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is primarily used to treat major depressive disorder and may also help with anxiety, sleep issues, and appetite loss.
Initial effects are seen within 1–2 weeks, with full therapeutic benefit usually achieved in 4–6 weeks.
Yes. Increased appetite and weight gain are common side effects, primarily due to antihistaminic activity.
Yes. Salius Pharma exports to international markets and operates WHO GMP, USFDA, UK MHRA, and TGA-approved facilities.
Yes. Complete DMF, CEP, impurity, stability, and CTD/ACTD dossiers are provided, with regulatory team assistance for global market registration.
Looking to source Mirtazapine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.