
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP
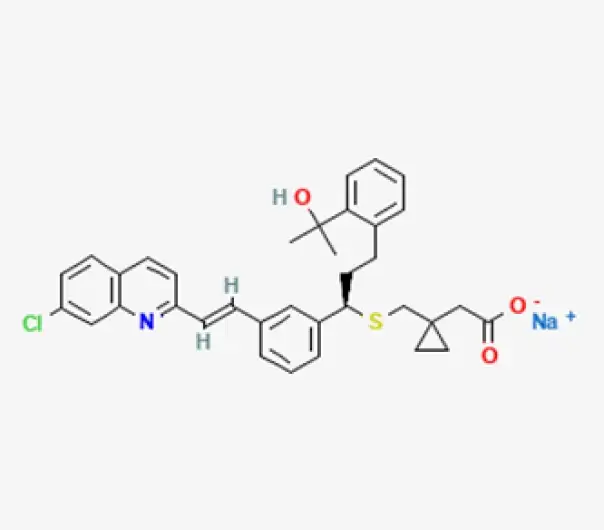
C35H35ClNNaO3S
[R-(E)]-1-[[[1-[3-[2-(7-Chloroquinolin-2-yl)vinyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt
151767-02-1
608.18 g/mol
Quinoline Derivative
Antiasthmatic
| appearance | White to off-white amorphous powder |
|---|---|
| solubility | Freely soluble in water and methanol; Practically insoluble in acetonitrile and ethanol |
| melting_point | 141–144°C |
| ph | 4.5 – 6.5 (1% aqueous solution) |
Montelukast Sodium is a selective leukotriene receptor antagonist used in the management of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. It blocks leukotriene-mediated airway inflammation and bronchoconstriction, improving airflow and reducing symptoms. With its high oral bioavailability, strong safety profile, and once-daily dosing, it is among the most widely used antiasthmatic APIs globally.
Montelukast selectively blocks cysteinyl leukotriene type 1 (CysLT1) receptors in the lungs and airways. By preventing leukotriene binding, it reduces airway edema, smooth muscle contraction, and mucus production, leading to improved respiratory function and reduced allergic inflammation.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used for the prevention and long-term treatment of asthma, and for relieving symptoms of seasonal and perennial allergic rhinitis.
It blocks cysteinyl leukotriene receptors (CysLT1) in the lungs and airways, reducing inflammation and preventing bronchoconstriction.
Headache, abdominal pain, and mild fatigue are common; mood changes and sleep disturbances are rare but possible.
Yes. Salius Pharma exports Montelukast Sodium worldwide with DMF and complete regulatory documentation support.
Yes. Full DMF, impurity profiles, COA, MSDS, stability data, and CTD-format dossiers are provided for registration in international markets.
Looking to source Montelukast Sodium or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.