
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP / BP / USP / EP
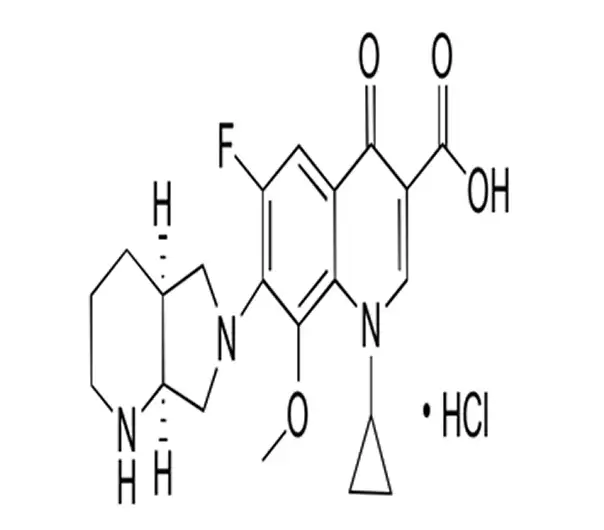
C21H24FN3O4·HCl
1-Cyclopropyl-7-[(S,S)-2,8-diazabicyclo[4.3.0]nonan-8-yl]-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride
186826-86-8
494.9 g/mol
Fluoroquinolone Antibiotic
| Appearance | Light yellow to pale orange crystalline powder |
|---|---|
| Solubility | Freely soluble in water; soluble in methanol and ethanol; poorly soluble in non-polar solvents |
| Melting point | 240–250°C (with decomposition) |
| pH | Stable in weakly acidic conditions (1% solution) |
Moxifloxacin Hydrochloride is a fourth-generation fluoroquinolone antibiotic with broad-spectrum activity against Gram-positive, Gram-negative, and atypical bacteria. The hydrochloride salt enhances its stability and handling properties, making it ideal for formulation into oral, parenteral, and ophthalmic dosage forms.
Moxifloxacin inhibits two key bacterial enzymes—DNA gyrase and topoisomerase IV—which are essential for DNA replication, transcription, and repair. Inhibition of these enzymes leads to disruption of bacterial DNA processes, causing cell death and preventing bacterial growth.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Moxifloxacin HCl has a plasma half-life of approximately 12–15 hours, allowing for convenient once-daily dosing.
Yes, it has excellent penetration into lungs, sinuses, skin, and eye tissues, with cerebrospinal fluid levels reaching 20–50% of plasma concentration.
Yes, it falls under HSE Category: Warning for potential genetic toxicity (H341). Appropriate safety measures should be followed during handling.
Salius Pharma exports Moxifloxacin HCl to the US, EU, Japan, Australia, Canada, and several emerging markets globally.
Salius Pharma holds WHO-GMP, ISO 9001:2015, and FDA inspection approvals across its manufacturing facilities.
Salius Pharma’s Star Export House status, regulatory documentation support, 99% on-time delivery rate, and competitive pricing make it a globally trusted API supplier.
Looking to source Moxifloxacin Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.