
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP
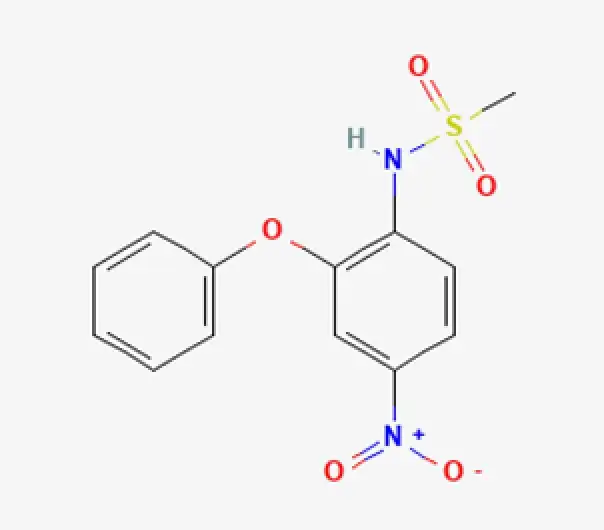
C13H12N2O5S
N-(4-nitro-2-phenoxyphenyl)methanesulfonamide
51803-78-2
308.31 g/mol
Non-steroidal anti-inflammatory compound (NSAID)
Analgesic • Antipyretic • Anti-inflammatory agent
| appearance | Yellow crystalline powder |
|---|---|
| solubility | Practically insoluble in water; soluble in acetone, ethanol, and methanol |
| melting_point | 138–141°C |
| ph | 5.0 – 7.0 (1% aqueous solution) |
Nimesulide is a selective COX-2–preferring NSAID providing effective relief from pain, fever, and inflammation with comparatively lower GI side effects. Manufactured under strict GMP for high purity and formulation compatibility.
Selectively inhibits COX-2, reducing prostaglandin synthesis to provide anti-inflammatory and analgesic effects while maintaining GI safety.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Low-impurity synthesis, assay >99.5%, stability, and controlled polymorph; batch-specific technical data available.
COA, MSDS, Packing List, Commercial Invoice, Batch Release; stability, impurity profile, and TDS on request.
CTD dossiers aligned with ICH Q3C/Q6A and pharmacopeial revisions for LATAM, MENA, CIS, and ASEAN.
Typically 2–3 weeks from order confirmation via GDP-compliant partners.
Multi-point in-process testing and full digital traceability from raw material to dispatch.
Formulation troubleshooting, method transfers, and regulatory clarifications.
Looking to source Nimesulide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.