
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / BP / EP
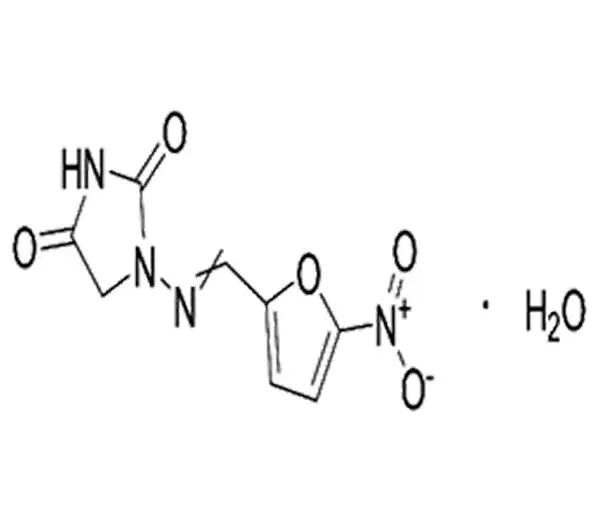
C8H8N4O5·H2O
1-(5-nitro-2-furfurylideneamino)hydantoin monohydrate
139-59-3
256.17 g/mol
Nitrofuran Antibacterial Agent
| Appearance | Yellow crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in DMSO; sparingly soluble in alcohol; pH-dependent solubility in acidic media |
| Melting point | ~250°C (decomposes) |
| pH | 5.0–7.0 (depending on formulation) |
Nitrofurantoin Monohydrate is a highly effective antibacterial API targeting urinary tract pathogens. As the hydrated crystalline form of Nitrofurantoin, it offers improved stability, dissolution, and controlled release properties. It is widely prescribed for acute and chronic urinary tract infections (UTIs) due to its strong urinary antibacterial action and low resistance rates.

Nitrofurantoin enters bacterial cells via passive diffusion, where bacterial flavoproteins reduce it to reactive intermediates. These intermediates attack DNA, RNA, and cell-wall proteins, leading to bactericidal effects specifically in urinary tissues. This multi-site mechanism minimizes the development of bacterial resistance.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is reduced by bacterial enzymes to reactive intermediates that damage DNA, RNA, ribosomal proteins, and metabolic enzymes, resulting in potent bactericidal activity in urinary tract tissues.
Primarily Gram-negative and some Gram-positive pathogens such as E. coli, Klebsiella (selected strains), Enterococcus faecalis, and Staphylococcus saprophyticus.
Yes. The monohydrate form allows slower dissolution and is commonly used in extended-release (Macrobid-type) capsule formulations.
It is urinary-specific. While active against multiple pathogens, its antibacterial activity is localized to the urinary tract due to rapid renal excretion.
Orders are typically dispatched within 7–14 business days, depending on documentation and QC clearance.
Short-term use is standard. For long-term prophylaxis, periodic monitoring of liver and lung function is advised.
Looking to source Nitrofurantoin Monohydrate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.