
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
EP/USP
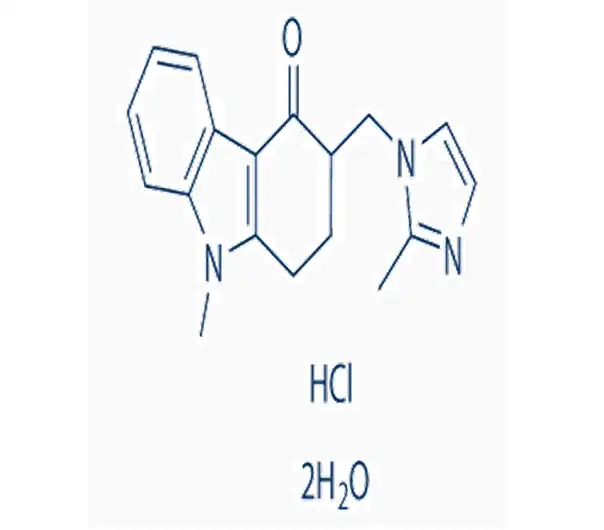
C18H19N3O·HCl·2H2O
(5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl}-1H-imidazole-5-carboxylate
103639-04-9
393.9 g/mol
Selective 5-HT₃ receptor antagonists
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Soluble in water; slightly soluble in methanol |
| Melting point | ~180–183 °C |
Ondansetron hydrochloride dihydrate prevents nausea and vomiting caused by chemotherapy, radiation, or surgery.
Ondansetron blocks serotonin 5-HT3 receptors in the brain and GI tract, preventing nausea.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015 |
| Export Experience | Proven global supplier |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
The dihydrate form includes water molecules within its crystal structure, which can improve the drug’s stability, solubility, and manufacturability compared to the anhydrous form.
Yes, while primarily approved for chemotherapy- and surgery-induced nausea, it is sometimes used off-label for nausea caused by other conditions such as gastroenteritis or pregnancy-related nausea, but only under medical supervision.
Ondansetron blocks 5-HT3 serotonin receptors specifically, preventing nausea signals, while metoclopramide works by blocking dopamine receptors and increasing gastrointestinal motility, making their uses and side effect profiles different.
Salius Pharma supplies Ondansetron HCl Dihydrate as an Active Pharmaceutical Ingredient (API).
To order Ondansetron HCl Dihydrate API, you typically contact a certified pharmaceutical supplier like Salius Pharma. You’ll need to provide details such as desired quantity, intended market, regulatory requirements (e.g., DMF or GMP certificates), and packaging preferences.
Salius Pharma exports Ondansetron HCl Dihydrate to various countries worldwide, including regions such as Asia, Europe, the Middle East, Africa, North America & South America.
Looking to source Ondansetron HCl Dihydrate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.