
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP / EP
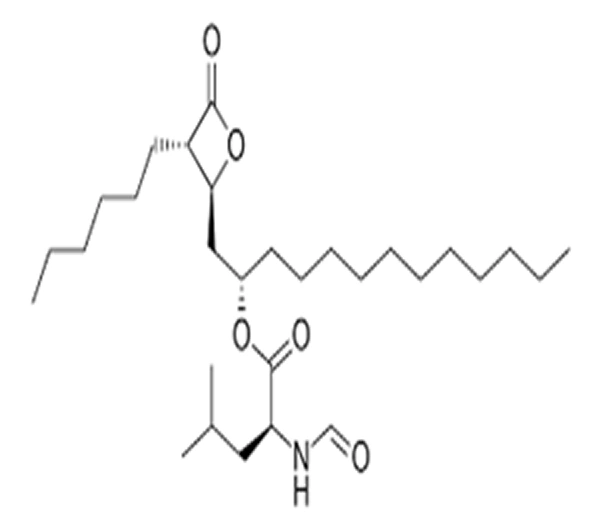
C₂₉H₅₃NO₅
( S )-((S)-1-((2S,3S)-3-Hexyl-4-oxooxetan-2-yl)tridecan-2-yl) N-formyl-L-leucinate
96829-58-2
495.7 g/mol
Lipase Inhibitor (Anti-obesity Agent)
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in dichloromethane; slightly soluble in ethanol; practically insoluble in water |
| Melting Point | 42–45°C |
| pH | Neutral |
Orlistat is a potent gastrointestinal lipase inhibitor used globally as an anti-obesity API. It reduces dietary fat absorption by inhibiting pancreatic and gastric lipases, preventing triglycerides from being hydrolyzed into absorbable free fatty acids. With proven clinical efficacy and favorable safety profile, Orlistat is widely used in prescription and OTC weight-management formulations.
Orlistat acts locally within the gastrointestinal tract by: Binding to gastric and pancreatic lipases; Preventing hydrolysis of dietary triglycerides; Reducing fat absorption by ~30% at therapeutic doses; Lowering total caloric intake. This results in measurable reductions in body weight, waist circumference, LDL cholesterol, and improve metabolic parameters.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Used for weight management and reducing dietary fat absorption in obese and overweight patients.
API grade compliant with BP/USP/EP standards.
Yes. We export across Asia, Europe, Africa, Middle East, North & South America with full regulatory and logistics support.
Salius Pharma is a WHO-GMP certified pharmaceutical manufacturer and global exporter specializing in APIs, intermediates, nutraceuticals, and finished formulations.
Each batch undergoes: Analytical testing; Impurity profiling; Microbial testing; Stability studies; In-process QC checks; Final QA release
Delivery is usually completed in 7–21 business days depending on stock availability, documentation, and destination
Looking to source Orlistat or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.