
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP
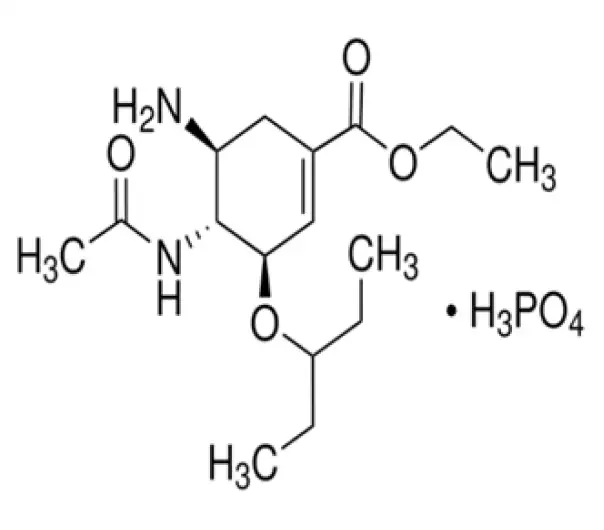
C16H28N2O4·H3PO4
(3R,4R,5S)-4-Acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1)
204255-11-8
410.42 g/mol
Carboxylate ester
Antiviral agent
| Appearance | Yellow crystalline powder |
|---|---|
| Solubility | Freely soluble in water and methanol; slightly soluble in ethanol; practically insoluble in acetone and ether |
| Melting Point | 186 – 191°C |
| pH (1% aqueous solution) | 5.0 – 6.5 |
Oseltamivir Phosphate is a potent neuraminidase inhibitor used worldwide for the treatment and prevention of influenza A and B infections. It blocks the release of viral particles from infected cells, reducing both the severity and duration of influenza symptoms.
Oseltamivir Phosphate is a prodrug that is converted in the liver to its active metabolite, oseltamivir carboxylate. The active form selectively inhibits viral neuraminidase enzymes, preventing the release and spread of new viral particles from infected host cells.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Manufactured under GMP and ICH Q7 guidelines with strict control on polymorphic form, purity (>99.5%), and residual solvents to meet BP/USP standards.
Yes. We supply both pharmaceutical-grade and government tender-grade API with full COA, TDS, and traceability documentation.
We provide CTD/ACTD dossiers and DMF open parts for registrations in regulated and semi-regulated markets, with regional adaptations for authorities like ANVISA, COFEPRIS, and SFDA.
Oseltamivir Phosphate is stable at ambient temperature; shipments to high-humidity regions are protected using desiccants, vacuum-sealed liners, and thermal packaging validated for Zone IVB.
Yes. The facility incorporates solvent recovery, low effluent discharge, and energy-efficient synthesis processes supporting environmental sustainability.
Yes. Samples (5–25 g) with COA and TDS are available. Full documentation (DMF, stability, CTD) can be shared under NDA for evaluation and registration.
Looking to source Oseltamivir Phosphate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.