
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP / EP
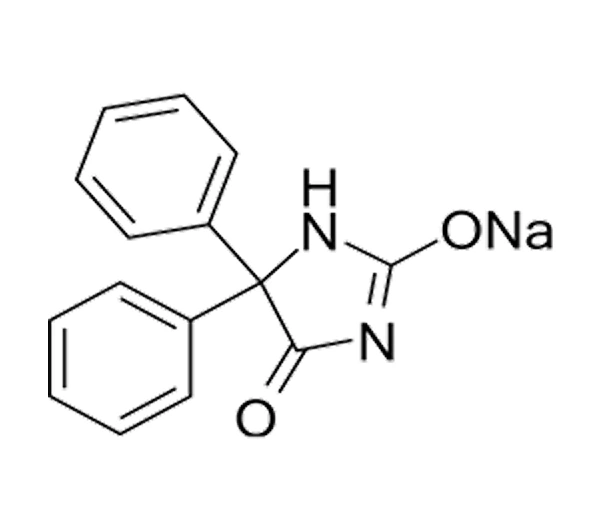
C15H11N2NaO2
Sodium 5,5-diphenylimidazolidine-2,4-dione
630-93-3
274.25 g/mol
Hydantoin Derivative – Anticonvulsant API
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water; sparingly soluble in alcohol |
| Melting Point | 278–283°C (with decomposition) |
| pH | 10.0 – 12.0 (alkaline) |
Phenytoin Sodium is a widely used anticonvulsant API essential in the treatment of epilepsy, tonic–clonic seizures, and status epilepticus, and is also used as an antiarrhythmic agent. It stabilizes neuronal membranes and reduces seizure impulses by modulating sodium ion transport across nerve cells.
Phenytoin Sodium works by slowing the rate of sodium channel recovery during nerve depolarization, reducing repetitive neuronal firing, stabilizing hyperexcitable neural tissue, and preventing seizure propagation. This makes it highly effective for long-term epilepsy management.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes. We work with clients on forecast planning, inventory planning, and annual supply agreements to ensure uninterrupted manufacturing.
Yes. Labels can be printed in English, French, Spanish, Arabic, Russian, or buyer-specified languages.
Yes. Method validation parameters like accuracy, specificity, linearity, and robustness can be provided.
Yes. Custom labels, drum sizes, palletization, and language-specific labels can be provided.
WHO-GMP certified production, assured availability, international export expertise, competitive pricing, and strong regulatory backing
Delivery is usually completed in 7–21 business days depending on stock availability, documentation, and destination
Looking to source Phenytoin Sodium or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.