
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP / EP
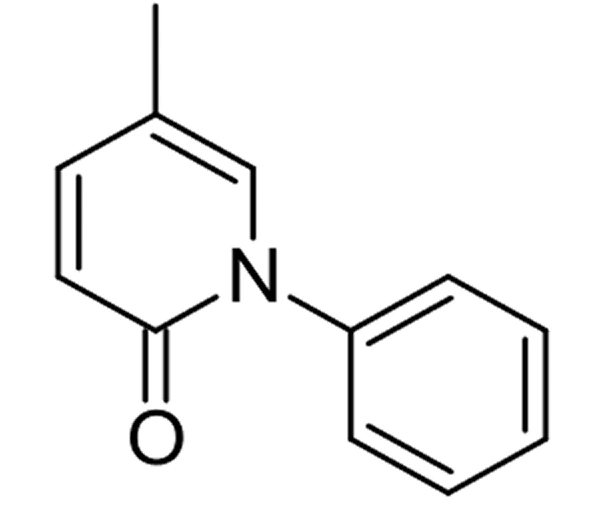
C12H11NO
5-Methyl-1-phenyl-2(1H)-pyridone
53179-13-8
185.22 g/mol
Pyridone Derivative – Anti-fibrotic API
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in DMSO & ethanol |
| Melting Point | 108–110°C |
Pirfenidone is a novel anti-fibrotic API used primarily for the treatment of Idiopathic Pulmonary Fibrosis (IPF). It reduces fibroblast proliferation, cytokine production, and collagen synthesis, thereby slowing the progression of pulmonary fibrosis.
Pirfenidone works by inhibiting TGF-β (Transforming Growth Factor-β), reducing collagen synthesis, suppressing fibroblast proliferation, and reducing inflammatory cytokines such as TNF-α.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Because we offer high-purity GMP-certified API, strong regulatory support, competitive pricing, fast, reliable export logistics, transparent documentation, and consistent supply capacity.
Yes. Our Pirfenidone complies with USP, BP, and EP standards based on buyer requirements.
Yes. Method validation parameters like accuracy, specificity, linearity, and robustness can be provided.
Yes. Custom labels, drum sizes, palletization, and language-specific labels can be provided.
Store below 30°C, in a tightly sealed, light-protected container.
Delivery is usually completed in 7–21 business days depending on stock availability, documentation, and destination
Looking to source Pirfenidone or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.