
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP
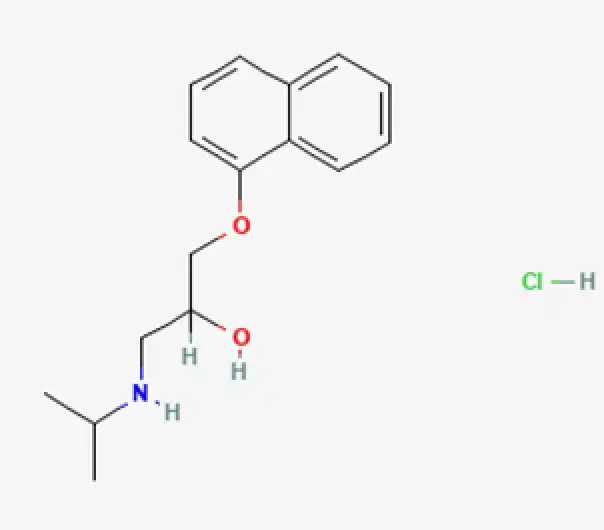
C16H22ClNO2
(RS)-1-(Isopropylamino)-3-(1-naphthyloxy)propan-2-ol hydrochloride
318-98-9
295.81 g/mol
Naphthoxypropanolamine derivative
Antihypertensive agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, methanol, and ethanol; slightly soluble in chloroform; insoluble in ether |
| Melting Point | 163–166°C |
| pH (1% aqueous solution) | 4.5 – 6.5 |
Propranolol Hydrochloride is a non-selective beta-adrenergic receptor blocker used for hypertension, angina pectoris, cardiac arrhythmias, and anxiety-related tachycardia. It remains a cost-effective cornerstone therapy in cardiovascular care.
Propranolol competitively blocks beta-1 (β1) and beta-2 (β2) adrenergic receptors, reducing heart rate, myocardial contractility, and cardiac output, while suppressing sympathetic nervous system stimulation. This results in lower blood pressure and decreased cardiac workload.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Manufactured under WHO-GMP and ICH Q7 guidelines with validated synthesis and impurity profiling to meet pharmacopeial standards.
Yes. We offer micronized and non-micronized grades tailored for tablets, capsules, and liquid dosage forms to ensure desired dissolution properties.
Our API is registered and supplied across LATAM, MENA, CIS, and Southeast Asia with CTD/ACTD dossiers and full analytical documentation.
Each batch is packed in HDPE drums with double PE liners, optionally nitrogen-flushed for stability. Shipments follow GDP with COA, MSDS, and TDS enclosed.
Each lot undergoes in-process monitoring, HPLC and GC assays, moisture and stability testing (Zone IVa/IVb) to ensure consistent purity and potency.
Yes. DMF open parts, stability data, and specifications are available prior to commercial agreement for evaluation and registration support.
Looking to source Propranolol Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.