
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP / BP / USP
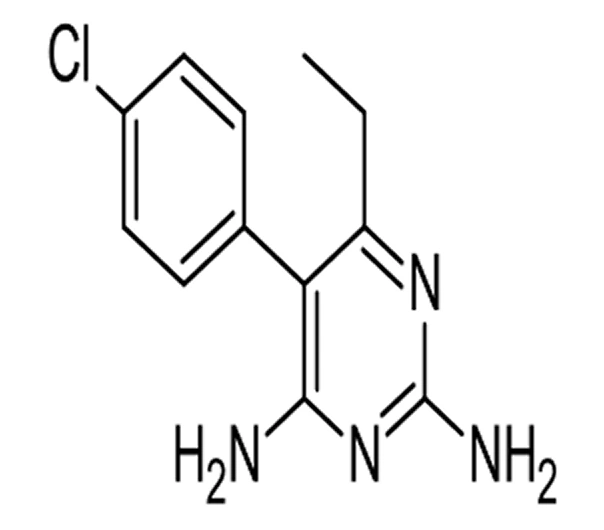
C12H13ClN4
5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine
58-14-0
248.71 g/mol
Antiprotozoal / Antimalarial Agent
| Appearance | White to yellowish crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; slightly soluble in alcohol and organic solvents |
| Melting Point | 233–235°C |
| pH (1% solution) | Neutral |
Pyrimethamine is a potent antiprotozoal agent widely used in the treatment and prevention of malaria as well as toxoplasmosis. It works synergistically with sulfonamides and is included in several fixed-dose combinations used in malaria-endemic regions.It is known for its long half-life and strong activity against Plasmodium species, especially when paired with a sulfa drug.
Pyrimethamine selectively inhibits dihydrofolate reductase (DHFR) in protozoa, preventing folic acid synthesis. This disrupts DNA replication and cell division, ultimately killing the parasite.It shows potent activity against Plasmodium falciparum, P. vivax, and Toxoplasma gondii.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is primarily used for malaria treatment and prevention, and for toxoplasmosis in combination therapy.
Yes, custom purity levels and specifications can be arranged.
COA, MSDS, GMP certificate, COO, packing list, commercial invoice, and additional documents as required.
Yes. We provide COA, MSDS, GMP certificates, packing list, commercial invoice, COO, and all compliance documents needed for smooth international customs clearance.
All documents are usually prepared within 24–72 hours after product QC approval and packaging.
Looking to source Pyrimethamine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.