
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
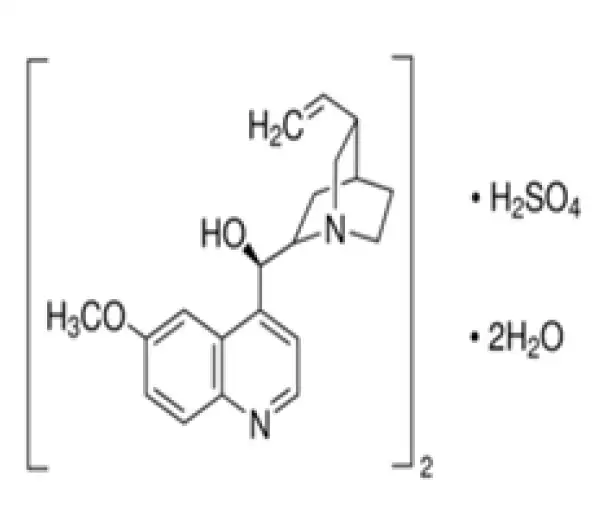
(C₂₀H₂₄N₂O₂)₂ · H₂SO₄ · 2 H₂O
[(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo2.2.2octan-2-yl]-(6-methoxyquinolin-4-yl)methanol; sulfuric acid]
804-63-7
782.94 g/mol
Antimalarial
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water |
| Melting point | 205–225°C |
| pH | 4.5–6.5 |
Quinine Sulfate is a medication primarily used to treat malaria, especially when caused by Plasmodium falciparum, the most dangerous type of malaria parasite. It’s also sometimes used to treat nighttime leg cramps, although this is less common due to potential side effects.

Quinine kills malaria parasites by inhibiting hemozoin formation, causing a toxic buildup of free heme inside the parasite. This leads to oxidative damage and parasite death.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is primarily used to treat malaria caused by Plasmodium falciparum, and occasionally prescribed for nocturnal leg cramps, though such use is rare today.
It interferes with hemozoin formation inside the parasite, leading to accumulation of toxic heme that damages and kills the parasite.
Yes, ringing in the ears (tinnitus), dizziness, and nausea are common side effects, particularly at higher doses.
Salius Pharma supplies Quinine Sulfate as an Active Pharmaceutical Ingredient (API).
To order, contact Salius Pharma with your required quantity, target market, and documentation needs (DMF, GMP, stability data, etc.).
Salius Pharma exports Quinine Sulfate globally, including Asia, Europe, Africa, the Middle East, North America, and South America.
Looking to source Quinine Sulfate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.