
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
Pharmaceutical Grade
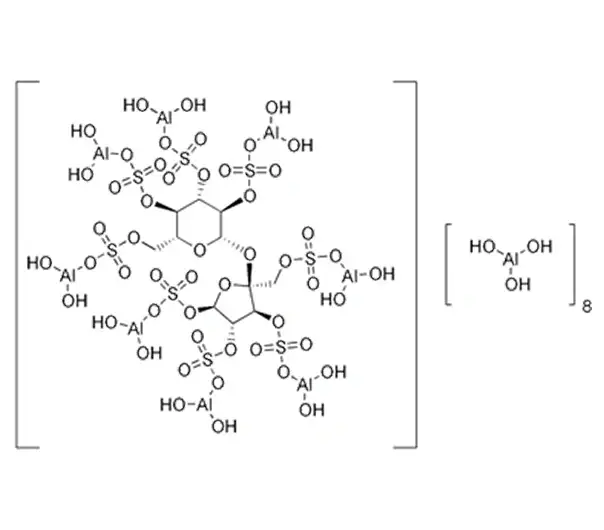
C12H54Al16O75S8
Basic aluminum sucrose sulfate
54182-58-0
2086.9 g/mol
Basic aluminum salt of sucrose octasulfate
Antiulcer / Gastroprotective agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Insoluble in water and ethanol; dispersible in acidic media |
| Melting Point | 178 – 182°C (with decomposition) |
| pH (1% aqueous solution) | 4.0 – 5.0 |
Sucralfate is a gastroprotective agent used to treat and prevent ulcers in the stomach and duodenum. It works locally by forming a viscous, adhesive barrier over ulcerated tissue, shielding it from acid, pepsin, and bile salts. With minimal systemic absorption, Sucralfate is considered safe, well-tolerated, and highly effective in managing peptic ulcer disease, gastritis, and reflux-related mucosal injury.
Sucralfate acts primarily through local, surface-protective mechanisms rather than systemic effects. In acidic conditions (pH < 4), it polymerizes to form a viscous, adhesive paste that binds to positively charged proteins at the ulcer site. This forms a physical barrier that protects the lesion from gastric acid, pepsin, and bile salts. Additionally, it stimulates local prostaglandin and bicarbonate secretion, enhancing mucosal defense and promoting ulcer healing.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Exported to Latin America, Middle East, Africa, and Asia, depending on local regulatory approvals and import registrations.
Classified as an Active Pharmaceutical Ingredient (API) under HS Code 29420090 – Other organic compounds (pharmaceutical use).
Yes, in semi-regulated markets. For regulated markets, product registration or inclusion in local dossiers may be required.
No special handling is required. Sucralfate is non-hazardous for air and sea transport (IATA/IMDG compliant).
Yes. Neutral labeling, customized packaging, and private branding options are available per buyer or export requirements.
Common incoterms include FOB, CIF, or DAP, based on buyer’s logistics preference and destination.
Looking to source Sucralfate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.