
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP / BP / USP
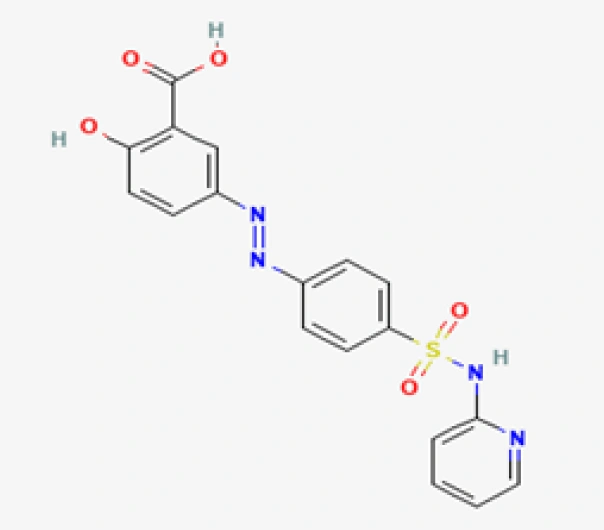
C18H14N4O5S
2-Hydroxy-5-[(2-pyridinylamino)sulfonyl]azo-benzene
599-79-1
398.39 g/mol
Azo-linked sulfonamide derivative
| Appearance | Orange-yellow to bright yellow crystalline powder |
|---|---|
| Solubility | Insoluble in water; soluble in NaOH solution |
| Melting Point | 250–260°C (decomposition) |
| pH | ~6–8 |
Sulfasalazine is an anti-inflammatory and immunomodulatory medication used primarily in the treatment of ulcerative colitis, Crohn’s disease, and rheumatoid arthritis. It consists of two pharmacologically active components—5-aminosalicylic acid (5-ASA) and sulfapyridine—joined by an azo bond that breaks down in the intestine to release both agents. Salius Pharma supplies high-quality Sulfasalazine API as an orange-yellow crystalline powder, compliant with IP, BP, and USP pharmacopeial requirements.
Inside the colon, intestinal bacteria break the azo bond of Sulfasalazine into: 5-ASA (Mesalamine): anti-inflammatory effect on the gut; Sulfapyridine: systemic immunomodulatory activity. Together, these actions reduce inflammation, suppress immune overactivity, and improve gastrointestinal symptoms.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
To treat ulcerative colitis, Crohn’s disease, and rheumatoid arthritis.
Yes. DMF support (if required), COA, MOA, stability data, and impurity profile are provided.
Standard PSD or customized micronization is available.
Yes. We export to Asia, Africa, Europe, the Middle East, LATAM, and CIS countries.
Yes, based on customer requirement.
You can email info@saliuspharma.com or WhatsApp +91 84258 80640 / 80643 with your required quantity and market.
Looking to source Sulfasalazine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.