
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP / BP / USP
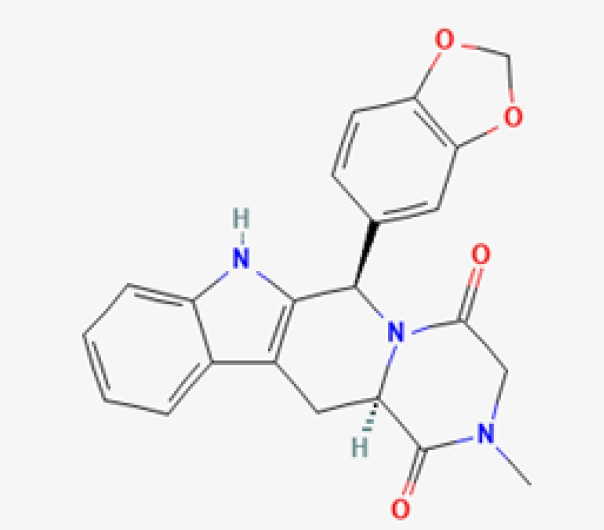
C22H19N3O4
(6R,12aR)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-1H-pyrazino [1,2:1,6] pyrido[3,4-b] indole-1,4-dione
171596-29-5
389.41 g/mol
Phosphodiesterase type-5 (PDE5) inhibitor
| Appearance | Off-white to yellow crystalline powder |
|---|---|
| Solubility | Insoluble in water; soluble in organic solvents |
| Melting Point | 299–302°C |
| pH | N/A (insoluble in water) |
Tadalafil is a potent, long-acting phosphodiesterase type-5 inhibitor widely used for the treatment of erectile dysfunction (ED), benign prostatic hyperplasia (BPH), and pulmonary arterial hypertension (PAH). It enhances smooth muscle relaxation by increasing intracellular cyclic GMP levels, resulting in improved blood flow. Salius Pharma supplies high-purity Tadalafil API meeting IP/BP/USP pharmacopeial requirements.
Tadalafil works by selectively inhibiting PDE5, an enzyme that breaks down cyclic guanosine monophosphate(cGMP). By allowing cGMP levels to increase, it promotes relaxation of smooth muscle tissues in the corpus cavernosum, prostate, bladder, and pulmonary arteries. This results in: Improved erectile function; Reduced symptoms of BPH; Lower pulmonary arterial pressure. Its long half-life of ~17.5 hours give Tadalafil a duration of action of 24–36 hours, earning it the name "weekend pill" in the ED segment.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It selectively inhibits PDE5, increasing cGMP levels and promoting smooth muscle relaxation.
Yes. DMF support (if required), COA, MOA, stability data, and impurity profile are provided.
Typically 36–48 months under recommended storage conditions.
Yes. We export to Asia, Africa, Europe, the Middle East, LATAM, and CIS countries.
Delivery usually takes 7–21 working days depending on destination and documentation.
You can email info@saliuspharma.com or WhatsApp +91 84258 80640 / 80643 with your required quantity and market.
Looking to source Tadalafil or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.