
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / EP / BP
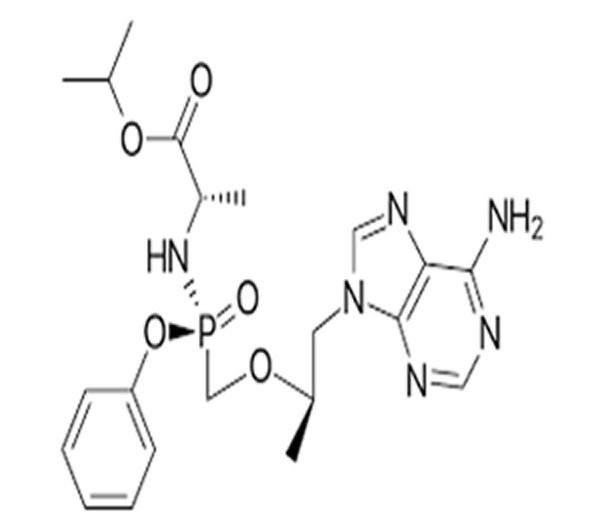
C₂₀H₂₈N₆O₅P
Isopropyl (2S)-2-[[[1R)-2-(6-amino-9H-purin-9-yl)-1-methoxypropan-2-yl] oxy] methyl-phenoxy phosphoryl] amino] propanoate
379270-37-8
476.5 g/mol
Nucleotide Reverse Transcriptase Inhibitor (NtRTI)
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in DMSO and methanol; slightly soluble in water |
| Melting Point | 105–110°C (decomposes) |
| pH | 4.5–6.5 (1% aqueous solution) |
Tenofovir Alafenamide is a novel nucleotide reverse transcriptase inhibitor used for the treatment of HIV-1 infection and chronic hepatitis B. It is a prodrug of tenofovir that delivers higher intracellular concentrations with lower systemic exposure, reducing the risk of renal and bone toxicity compared to Tenofovir Disoproxil Fumarate (TDF).
TAF is converted inside cells into tenofovir diphosphate. Compete with natural nucleotides to inhibit viral reverse transcriptase. Prevents viral DNA chain elongation, effectively reducing viral replication. Lower plasma tenofovir levels result in improved safety for kidneys and bones.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used for HIV-1 infection and chronic hepatitis B treatment.
Cool, dry, ventilated area; protect from light and moisture.
Yes.
Salius Pharma exports globally, including regulated markets in the US, EU, and Asia.
Yes, stability and analytical data are provided with COA.
Contact Salius Pharma via email or phone with product name, quantity, and delivery details; a quotation and documentation package will be provided.
Looking to source Tenofovir Alafenamide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.