
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / EP / IP
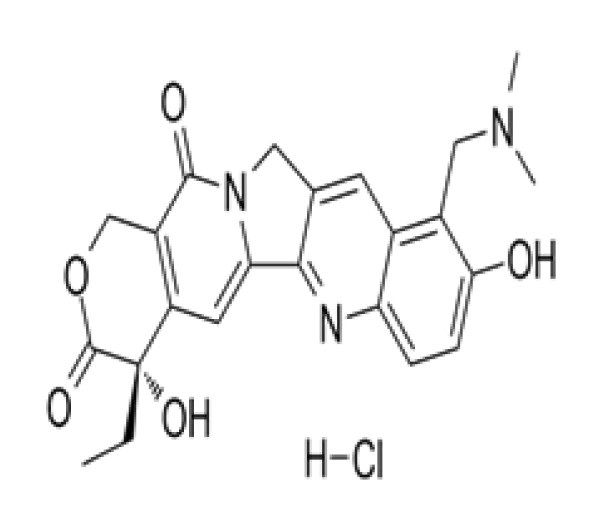
C23H23N3O5 · HCl
(4S)-4,11-diethyl-4-hydroxy-9-[4-piperidino) piperidino]methyl-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14-dione hydrochloride
119413-55-7
457.9 g/mol (approx.)
Camptothecin derivative; Topoisomerase I inhibitor (Antineoplastic agent)
| Appearance | Light yellow to yellow crystalline powder |
|---|---|
| Solubility | Freely soluble in water; soluble in methanol |
| Melting Point | Approx. 213–215 °C (with decomposition) |
| pKa | ~6.7 |
Topotecan Hydrochloride is a semi-synthetic camptothecin derivative used as an anticancer agent. It inhibits DNA topoisomerase I, leading to DNA damage and apoptosis in rapidly dividing tumor cells.
Topotecan selectively inhibits topoisomerase I by stabilizing the cleavable complex between the enzyme and DNA. This prevents DNA religation during replication, resulting in single-strand DNA breaks that trigger cell death, particularly in proliferating cancer cells.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes. It is a potent cytotoxic anticancer API and must be handled in dedicated oncology facilities with appropriate containment.
It is moderately hygroscopic and should be stored in moisture-resistant containers.
Yes. It is commonly used for injectable dosage forms and meets parenteral-grade requirements.
Compatibility studies are recommended, especially for pH-sensitive excipients.
Yes. It is supplied to multiple international markets in compliance with global regulatory expectations.
Looking to source Topotecan Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.