
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / EP / BP / JP
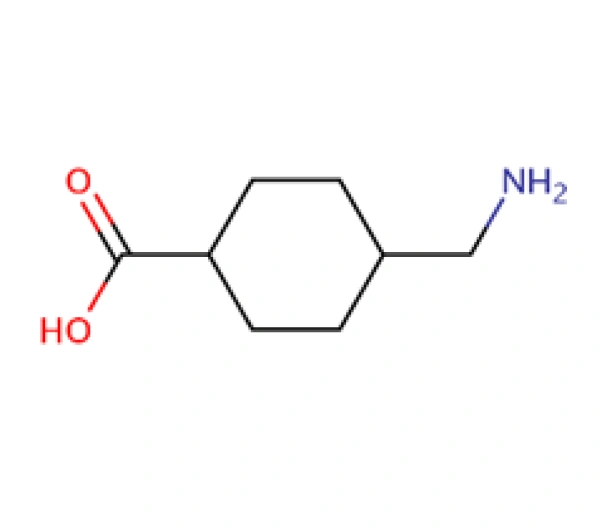
C₈H₁₅NO₂
trans-4-(Aminomethyl)cyclohexanecarboxylic acid
1197-18-8
157.21 g/mol
Antifibrinolytic
| Appearance | White or almost white crystalline powder |
|---|---|
| Solubility | Freely soluble in water; Slightly soluble in alcohol; Practically insoluble in acetone & chloroform |
| Melting Point | > 300°C (decomposes) |
| pH | 6.5 – 8.0 |
Tranexamic Acid is a synthetic anti-fibrinolytic agent widely used to control excessive bleeding in surgical, gynecological, trauma, and medical conditions. It inhibits the breakdown of fibrin clots, thereby helping maintain hemostasis.
TXA is a competitive inhibitor of plasminogen activation. It works by: Blocking the conversion of plasminogen to plasmin; Preventing fibrin degradation; Stabilizing blood clots; Reducing excessive bleeding. TXA is highly effective in surgery, trauma care, heavy menstrual bleeding, and dermatology.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used to prevent or reduce excessive bleeding in surgical, medical, gynecological, and trauma conditions.
Yes. Salius Pharma exports APIs, excipients, nutraceuticals, and cosmetic ingredients to over 50+ countries across Asia, Africa, the Middle East, Europe, and the Americas.
Typically 7–14 business days for stocked items and 2–4 weeks for custom or documentation-heavy products.
APIs are packed in HDPE drums, fiber drums, or sealed containers with double liners to ensure moisture control and transport stability.
Yes, the company provides technical dossiers, impurity profiles, stability data, and regulatory documentation upon request.
Yes, Salius supports SGS, Intertek, Bureau Veritas, and other third-party QC inspections upon request.
Looking to source Tranexamic Acid or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.