
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / BP
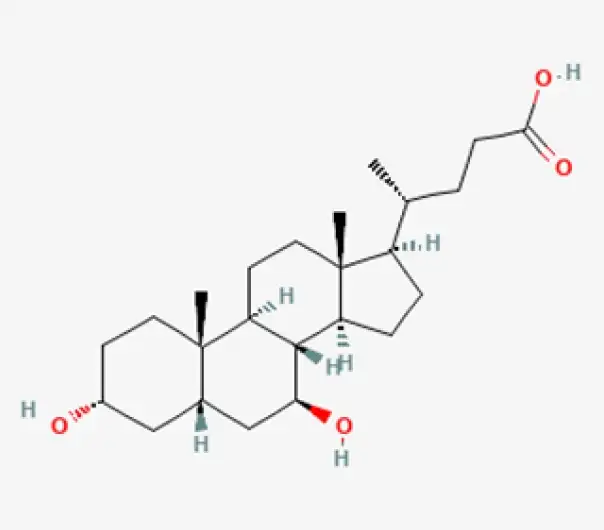
C24H40O4
(3α,5β,7β)-3,7-Dihydroxycholan-24-oic acid
128-13-2
392.57 g/mol
Secondary bile acid
Hepatoprotective agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; freely soluble in ethanol |
| Melting Point | 178 – 182°C (with decomposition) |
| pH (1% aqueous solution) | 4.0 – 5.0 |
Ursodeoxycholic Acid (UDCA) is a naturally occurring bile acid and a key hepatoprotective agent. It is used to dissolve gallstones, improve bile flow, protect liver cells, and reduce cholesterol saturation in bile. It is one of the most widely prescribed agents for cholestatic liver diseases and gallstone prevention.
Ursodeoxycholic Acid modifies the bile acid composition and reduces hepatocellular injury by decreasing toxic hydrophobic bile acids. It stabilizes hepatocyte membranes, protects mitochondria from oxidative stress, and promotes choleresis (bile secretion). UDCA also reduces cholesterol absorption and secretion into bile, preventing gallstone formation.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Pharmaceutical Grade conforming to USP / BP standards, suitable for tablet and capsule formulations.
Comprehensive documentation including CoA, MSDS, TDS, GMP Certificate, DMF (Open Part), Stability Data, and CTD dossier upon request.
Exported to LATAM, MENA, CIS, Africa, and Southeast Asia, with expanding regulatory approvals worldwide.
Yes. Our regulatory team prepares and customizes CTD/ACTD dossiers and analytical data per importing country requirements.
Dispatch within 10–15 working days depending on stock status and documentation readiness.
Packed in 25 kg fiber or HDPE drums with double polyethylene liners to ensure stability and compliance with international transport standards.
Looking to source Ursodeoxycholic Acid or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.